New website and updated pipeline for Gateway Biotechnology reveals their nasal spray (Nimodipine) and gene therapy tinnitus treatments are still advancing toward human trials.
Developing story…
Last fall, we shared Gateway Biotechnology’s updated pipeline graphic that was published without a press release, announcement, or social media posts. We just discovered that Gateway has changed their primary website domain, and further updated their tinnitus treatment pipeline.
The domain used to be gatewaybiotechnology.com and is now gatewaybiotechnology.net. We can only speculate as to why they chose to make this change…
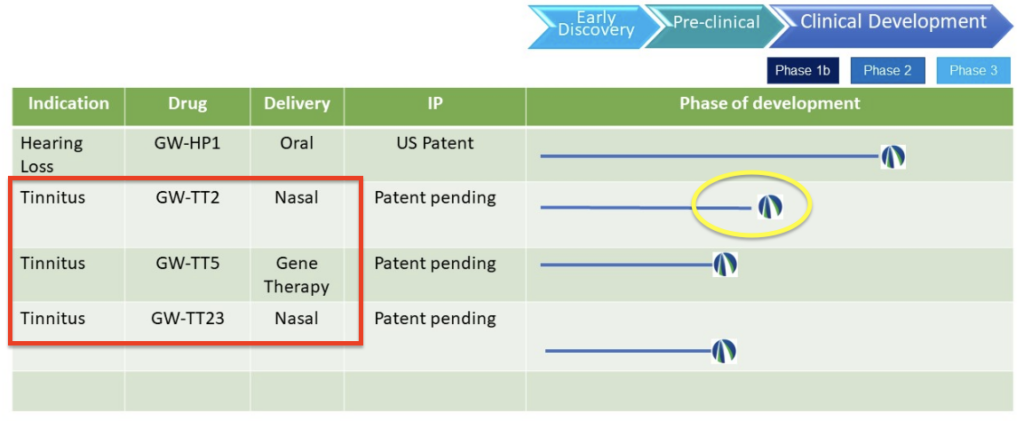
As for the tinnitus product pipeline, below is the screenshot of it from last fall that included four potential new products for tinnitus and hearing loss.
The September 2023 pipeline (on https://www.gatewaybiotechnology.com)…
As of this writing, Gateway’s focus is now on GW-TT2, a nasal spray for moderate to severe tinnitus, and GW-TT5, a gene therapy for severe, debilitating tinnitus.
The updated product pipelines for GW-TT2 and GW-TT5 (found on https://www.gatewaybiotechnology.net) are shown below.
GW-TT2 (nasal spray for moderate to severe tinnitus)
GW-TT2 is the nasal spray designed to treat moderate to severe tinnitus that we mentioned last year.
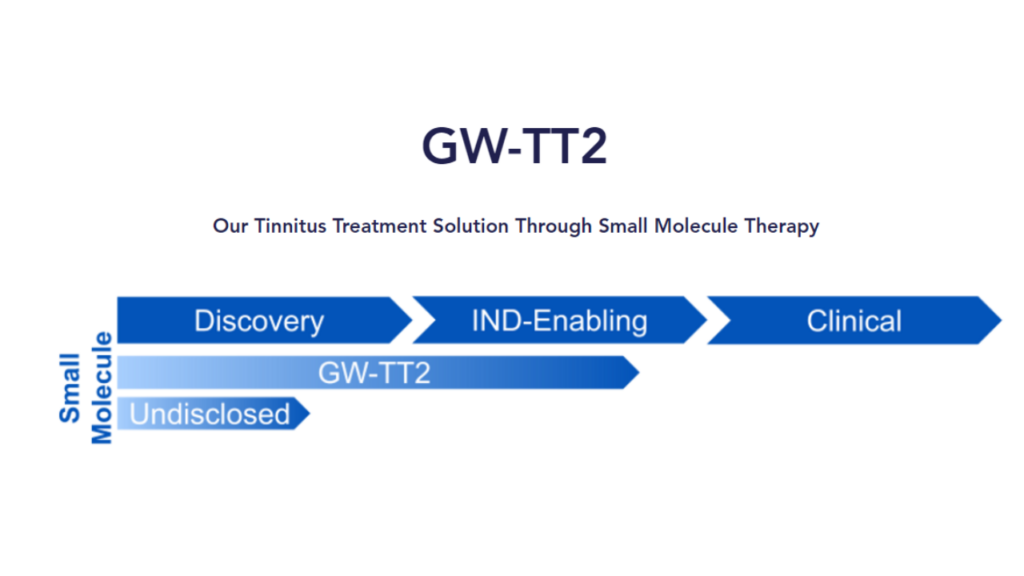
While Gateway says that this treatment is for recent onset tinnitus that has developed within the last two years, they go on to say,
“Recent onset tinnitus is the initial indication but expansion into additional indications is likely.”
May 28, 2024 update:
Today we are sharing more information about GW-TT2 (the nasal spray, which is a repurposed drug). This information is from the official Small Business Innovation Research (SBIR) program of the US government.
A deeper dive into GW-TT2 finds that the name of the drug is Nimodipine (NMDP). Here are the details from the official SBIR abstract for the project that began on September 18, 2023.
Agency: Department of Defense, Branch: Defense Health Agency
Accessed: 5-28-2024 13:37 p.m. EasternAbstract (emphasis ours)
” […] Despite extensive studies, no drugs have been approved by the U.S. Food and Drug Administration (FDA) for use in the amelioration of tinnitus. Exact pathological mechanisms for noise-induced tinnitus are unknown. The current view is that tinnitus can be induced by neural hyperactivity in the central auditory nervous system after peripheral deafferentation associated with acoustic trauma. This abnormal electrical activity may be the direct result of an increase in calcium channel activity. Recent data from our SBIR direct Phase II grant (R44DC018759) showed that L-type, but not T-type calcium channel blockers are effective in the treatment of tinnitus after acoustic trauma. One such blocker of L-type calcium channels is nimodipine (NMDP), an FDA-approved drug with an extensive record of safety and pharmacokinetic data. In the U.S., an NMDP oral formulation is used to reduce ischemic deficits after subarachnoid hemorrhage, and in Europe, an intravenous formulation is approved for the same indication to increase its delivery to the brain. Using an established animal model for tinnitus induced by noise exposure, we developed a new nasal formulation for NMDP (GW-TT2) to reduce its systemic side effects, increase its brain delivery, and improve patients’ experience with the use of this drug. Our hypothesis is that NMDP, when delivered nasally, can be a safe and effective therapy for noise-induced tinnitus. Most importantly, we have recently received positive FDA comments for our Type B Pre-Investigational New Drug (IND) application, which provided a detailed map for our next steps in developing GW-TT2 including guidance on the Chemistry, Manufacturing and Controls (CMC) for the final drug product. Here the objective is to manufacture GW-TT2 for clinical phase I/Ib trials. By working closely with our contract research organizations (CROs), we will: (1) test the feasibility and procedures to make GW-TT2 using non-cGMP (current good manufacturing practices) standards; (2) use the product for toxicity testing on animals; and finally, (3) make cGMP-grade GW-TT2 drug for stability testing and clinical studies. These studies will result in a final drug product suitable for GMP Tox studies and Phase I/Ib clinical studies. If successful, these studies will enable us to open an IND and initiate clinical trials with the goal of providing the first-ever FDA approved drug for tinnitus and address an urgent unmet need for military personnel with tinnitus.”
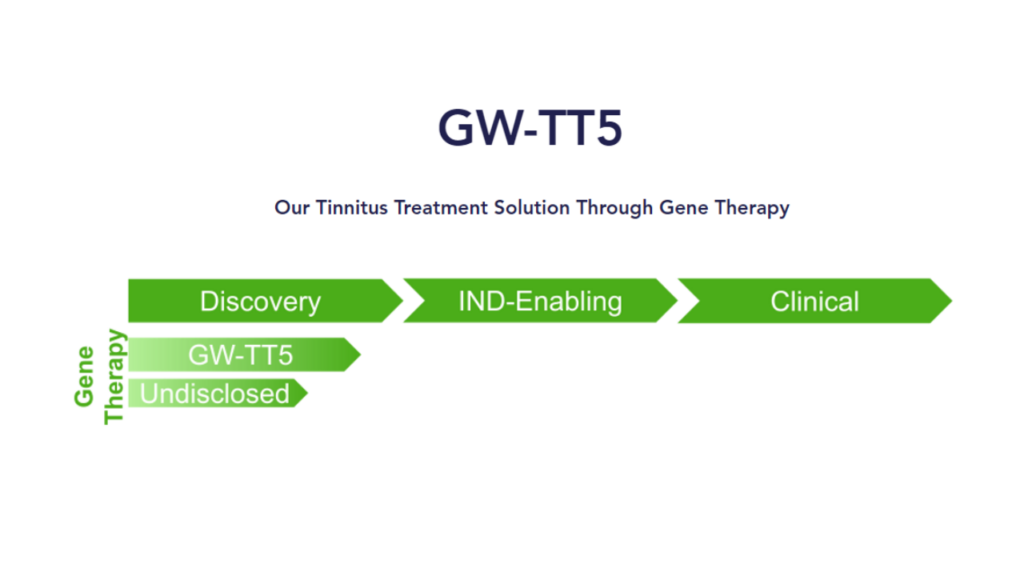
GW-TT5 (gene therapy for severe, debilitating tinnitus)
GW-TT5 is the gene therapy treatment for severe, debilitating tinnitus—for which there currently are no FDA-approved drugs. GW-TT5 is in the discovery phase, now in preclinical development.
When will GW-TT2 and GW-TT5 be available?
We also noted the progress of each of these drug candidates. Based on this new website, they’ve stayed on schedule where they last left off.
There seems to be an unearthed press release that we hadn’t seen that says what was happening with the pre-IND meeting with the FDA and how GW-TT2 was advancing toward clinical trials. With this press release, we have confirmation that they received a positive response regarding their plan CMC, non-clinical, and clinical development strategy and the FDA gave them a green light for their 505(b)(2) NDA-approval pathway, which means this candidate is going through an accelerated timeline.
How accelerated? What does that mean?
GW-TT2 (nasal spray) is still advancing toward human trials, with a phase 1 trial expected to start next year.
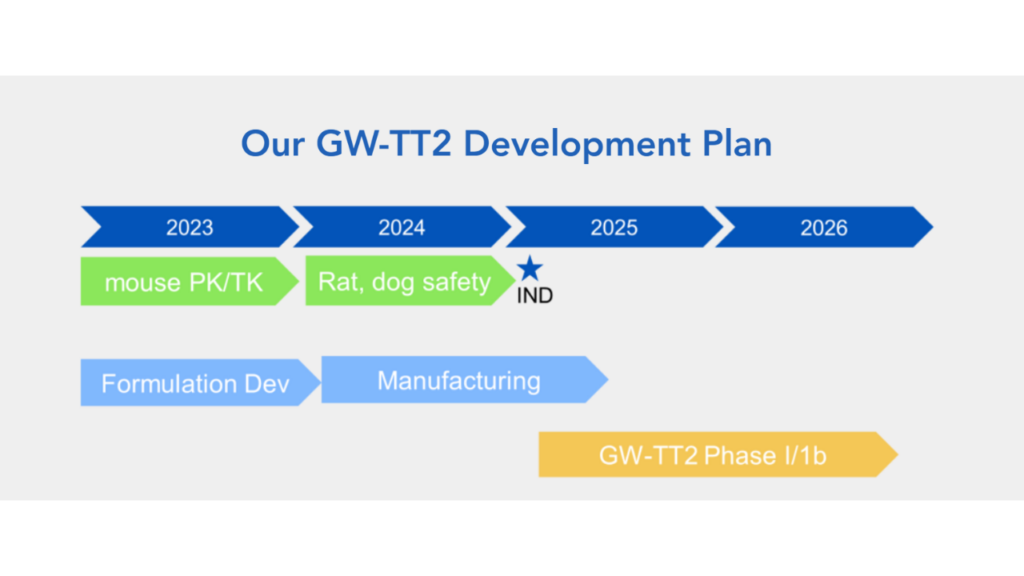
The official project end date for the research grant for GW-TT2 is January 17, 2026, according to our sources. This is consistent with a contract we learned about, and as of this writing has 1 year 8 months remaining. The research grant specifies GW-TT2 as a new product for tinnitus and has been awarded $1.3 million from the Defense Health Program. This is considered a medical research and development (R&D) contract and is an SBIR phase 2, which means that there is funding for the continuation of research. The phase 1 was successful enough, in the eyes of the Department of Defense, to continue funding their pursuit of this pathway.
GW-TT5 (the more interesting of the two) is a gene therapy targeting cells in the auditory pathway with apparently promising preclinical animal studies that suggest they’re “able to manipulate tinnitus symptoms with gene therapy.”
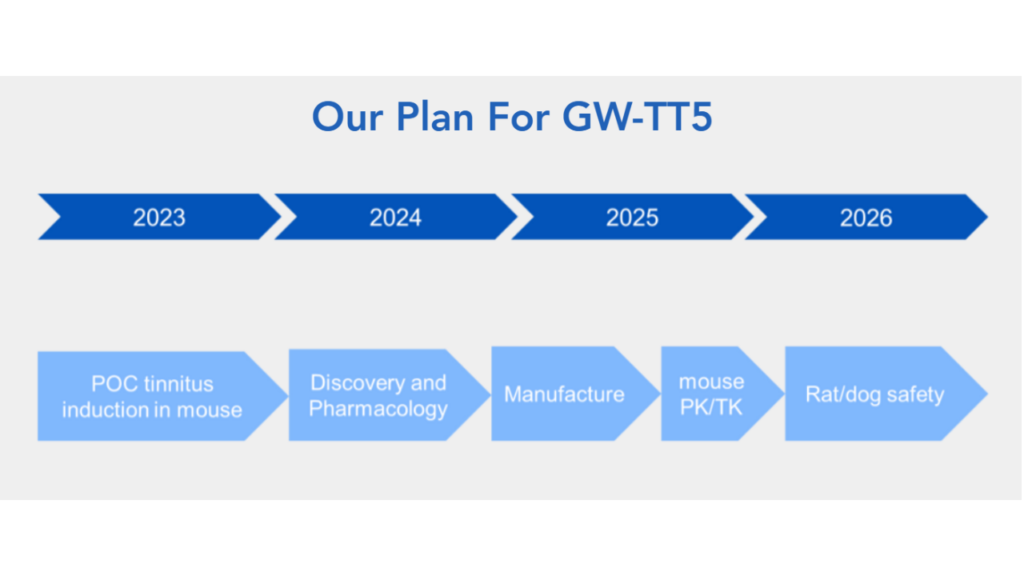
Now with Gateway’s new website and details added to it, we also know that GW-TT5, though not yet as far along as GW-TT2 (GW-TT5 is still in preclinical development), is intended for severe and debilitating tinnitus and targets a population of about 2 million people with tinnitus. Between now and the end of 2026, Gateway intends to complete safety studies in animal models and most likely follow-through with an IND submission to pave the way for human studies.
Genetic subtyping for tinnitus
What’s interesting, however, despite what may seem like yet another long wait for treatments in development, is a new section on the Gateway Biotechnology website that addresses tinnitus subtyping. Specifically, they mention that the ability to identify genetic signatures associated with genetic subtypes. GW-TT5 and a new series of objective tests could end up giving patients the knowledge of what tinnitus subtype they have and whether or not a gene therapy (such as GW-TT5, or what may emerge in the future) can impact their tinnitus.
To take it a step further, they add a list of other subtyping tests, such as metabolic profiling in blood samples that will allow them to define tinnitus subtypes and even non-invasive functional biomarkers related to audiometric data, and EEGs and MRIs that can allow them to zero-in on clinically relevant subtypes of tinnitus that are affecting people.
They now mention the “Gateway solution” which has subtying and therapies including small molecule and gene therapy as the solution.
There are other objective measures, but the DNA samples and gene variations seem most interesting given that Gateway also has their own gene therapy in the pipeline.
“By matching a given therapy to a defined subtype of tinnitus, we expect to see improved outcomes in clinical trials where therapies are tailored to a specific patient profile.”
Based on how this has played out in other chronic conditions, it’s perhaps the most needed and promising change and now we are seeing it play out with respect to a company’s commercial pipeline and what will follow.
What happened to the other treatment candidates (GW-TT23 & GW-HP1)?
In September, we shared an update regarding Gateway’s pipeline. At that time they listed both GW-TT2 and GW-TT5, but there were two other potential treatments as well, GW-TT23 for tinnitus and GW-HP1 for hearing loss. You can read our article on that here: https://www.tinnitustreatmentreport.com/gateway-biotechnology-adds-three-new-tinnitus-drugs-to-pipeline-gw-tt2-gw-tt5-gw-tt23/
Since then, it appears that GW-TT23 and GW-HP1 have been de-prioritized, or are no longer part of Gateway’s pipeline. Previously, these were mentioned on the company’s LinkedIn page and mentioned pre-clinical testing, but it appears they have either been discontinued because of the superiority of the other two candidates, or for other reasons (we don’t have any additional information, but it looks like they’re concentrating their efforts on the most promising candidates).
Overall, it appears that the most promising candidates from 10+ years of NIH-funded testing & 12+ drug combinations are GW-TT2 & GW-TT5, and these are what Gateway is now focusing on.
This is a developing story…
Article in progress.
How to get email updates on GW-TT2 and GW-TT5
If you want updates related to these two top treatments in development from Gateway Biotechnology — including the status of human clinical trials — sign up for the Tinnitus Treatment Report email newsletter.
It’s free, your information is kept private, and you can unsubscribe at any time with a single click. Best of all, as a subscriber, you get early access to exclusive updates like this one — before they are added to the front page of this website.
Expect between 1-2 emails per week… but only if something interesting and treatment-related is happening.
References
https://www.tinnitustreatmentreport.com/gateway-biotechnology-adds-three-new-tinnitus-drugs-to-pipeline-gw-tt2-gw-tt5-gw-tt23/
https://www.gatewaybiotechnology.com/services/ (old link)
https://www.gatewaybiotechnology.net/gw-tt2
https://www.gatewaybiotechnology.net/gw-tt5
https://www.gatewaybiotechnology.net/tinnitus-subtyping