New (and unexpected) phase 2 study will investigate whether “SAGE-547”, delivered as a 6-hour continuous IV infusion, can reduce tinnitus loudness, annoyance…
The study titled, An Open-Label Study Evaluating Brexanolone in Adults With Tinnitus, was added to the ClinicalTrials.gov database earlier today (December 9, 2022). Sage anticipates a March 2023 start date. So, although the trial status is currently set to “Not yet recruiting”… that could change any day now, and become increasingly likely as we approach March (less than 90 days from today).
The long-form title of the study is An Open-Label Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Brexanolone in the Treatment of Adult Participants With Tinnitus. Efficacy, check. Pharmacokinetics, check. Additional information from the NCT05645432 study record:
The primary objective of this study is to evaluate the safety and tolerability of brexanolone in participants with tinnitus following a single 6-hour continuous intravenous (IV) infusion.
This is a “rapid release” article draft.
So, no deep dive on SAGE-547 just yet. But perhaps what is most interesting right now is the TOTAL LACK of background information on SAGE-547 (Brexanolone delivered as a continuous 6-hour IV infusion) for tinnitus, specifically, on the Sage Therapeutics website.
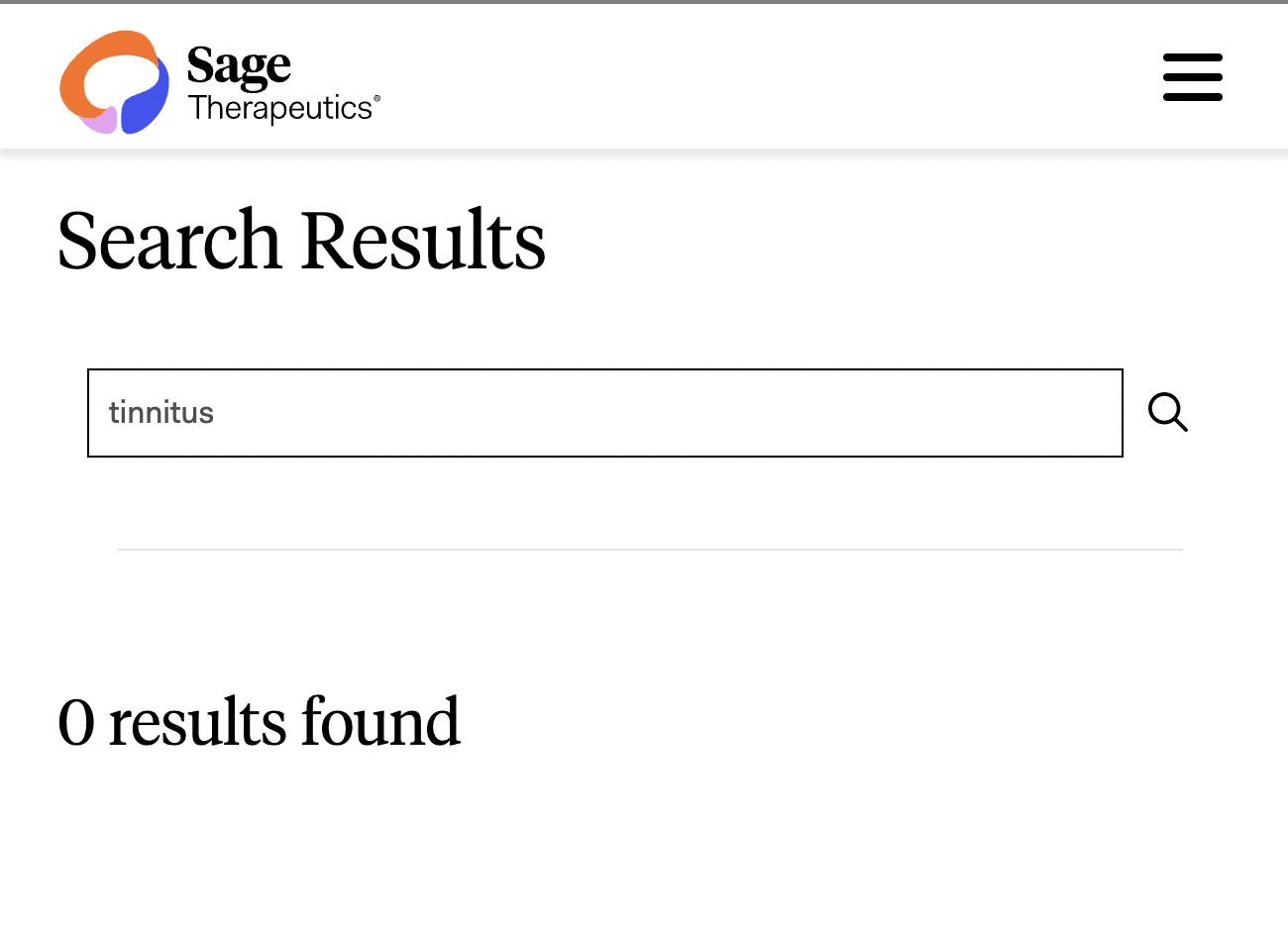
Why would a company developing a tinnitus drug and on the brink of initiating a phase 2 clinical trial in the next 90 days or so… have no mention of the condition on their website?
Was it a stealth candidate, for some reason? Did they discover something in another trial that got their attention? Is there some scientific article that suddenly made them realize they had something? Or could it have something to do with the patent they got in May? To be determined. Developing story…
The connection is undeniable…
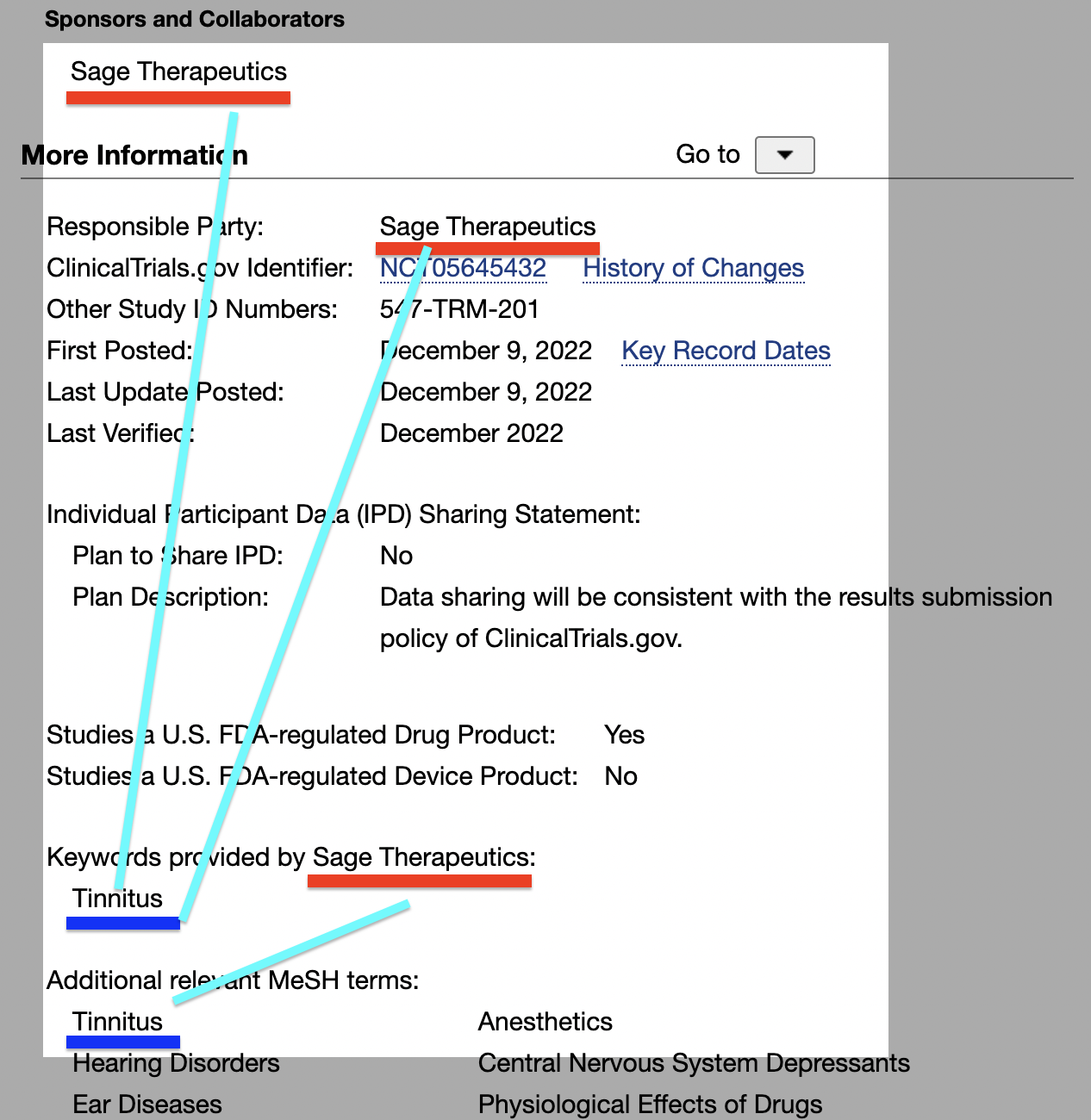
Undeniable…
In theory, I thought those screenshots were dramatic and powerful. In practice, they are ridiculous.
But, if nothing else, they are a reminder of how the treatment journey is full of surprises…
The truth is, we never quite know what emerging or promising or unexpected tinnitus drug, biotech company, or phase 2 trial is right around the corner.
… including times when that particular tinnitus treatment may still be under the radar or unannounced by the company developing it.
Speaking of developing… Looking further ahead, the anticipated completion date of this trial is September 2023.
Between ~ March and that time (around 6 months), researchers will be checking a variety of outcome measures, including tinnitus loudness (yay) and tinnitus annoyance, etc.
That’s all for now. THIS IS A DRAFT ARTICLE FOR EMAIL SUBSCRIBERS. MORE INFO TO FOLLOW. For updates and to do your own digging, as you wait…
You can read the official NCT05645432 study record (see link below) for the technical details:
https://www.clinicaltrials.gov/ct2/show/NCT05645432
As for the treatment and sponsor behind this new “6-hour continuous infusion” treatment. From the Sage Therapeutics official company website:
https://www.sagerx.com/search#stq=tinnitus
(I will do some digging and send a follow-up on what this drug is about in an upcoming issue. Do not let the tinnitus-word-less corporate website discourage you.)
How to get email updates on the development of SAGE-547 for tinnitus
“We believe the Test Treatment Report email newsletter is the fastest, most reliable way to get the latest updates on Sage’s new continuous IV infusion drug (plus many other promising new tinnitus treatments in development).”
Sign up for free, here: subscribe to email updates.